Ammonia Production in General
Ammonia is a gas consist with nitrogen and hydrogen with the composition NH3. It’s a colorless gas with a distinct strong odor. Ammonia is the world’s second-largest chemical product, including use in agriculture, food preparation, manufactured materials, coolants, and additives. Because of its higher hydrogen volume, low storage pressure, good stability for prolonged storage, high autoignition heat, and low condensing pressure, and lower gas density than air, the use of ammonia as an energy carrier has recently piqued attention.
In general, Ammonia was produce by using following processes,
- Electrochemical process
- Haber process
- Photochemical process
Although the most efficient manufacturing of ammonia, several techniques have been developed, The Haber process is mostly used to produce ammonia, in which nitrogen and hydrogen react in the existence of an iron catalyst to make ammonia.
Haber process
The Haber–Bosch process (Haber process) is one of the most effective and successful industrial processes for producing ammonia. In this ammonia is produce by reacting atmospheric nitrogen (N2) with hydrogen (H2) in the Haber process. This method are used to produce 85%of total of total ammonia global production. The synthesis of ammonia takes place in accordance with the reaction.
Raw materials use in the Haber process
- Nitrogen from the atmospheric air
- Hydrogen from the natural gas and steam

Ammonia synthesis is a negative enthalpy change exothermic reaction that happens naturally at low temperatures. Although it is preferred at room temperature, the rate of the reaction at room temperature is too sluggish to be practical on a large scale. A metal catalyst is utilized in this process, which is carried out at high temperatures and pressures. Because high temperature and pressure are needed to improve the kinetic of this reaction to obtain desired conversion rate.

The Haber process requires,
- Pressure : 200 – 400 atm
- Temperature : 450 – 500 0C
NH3 is continuously eliminated as ammonia is created in commercial production. According to Le Chatelier’s principle, eliminating the products causes additional nitrogen and hydrogen to mix.
Electrochemical process
The Haber process also has a low energy efficiency, with an NH3 rate of exchange of less than 15% each cycle (thermodynamically limited). The need for NH3 keeps rising in response to the world’s rising population. As a result, for both scientific industrial and research uses, a high efficiency, mild, sustainable, and environmental friendly alternative strategy to manufacture NH3 is critical.
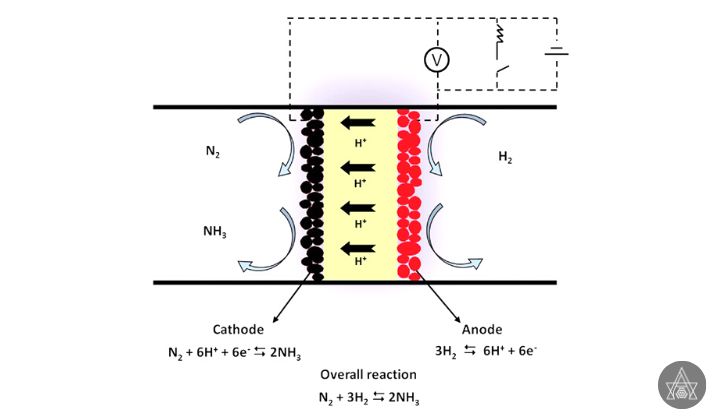
The reaction of N2 with a proton source under mild conditions generated from renewable electricity can produce electrochemical NH3, which is a viable carbon-neutral and sustainable solution. N2, on the other hand, has a great thermodynamic stability and requires a lot of energy to activate. Implementing this “clean” NH3 synthesis pathway will still necessitate major high level of energy efficiency, exchange rate, and longevity, which can only be achieved by designing efficient electro catalysts.
Two benefits of using an electrochemical method include the ability to produce ammonia at room temperature and pressure and the use of water as a source of protons. This process might potentially use less energy, be less expensive, and be more environmentally benign than the Haber method.
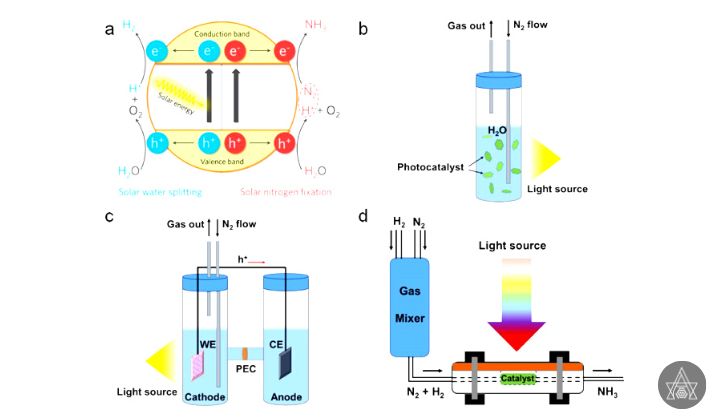
Photochemical process
Ammonia is produced industrially via the Haber-Bosch process, which involves high temperatures and pressures. Some enzyme can transform N2 to NH3 at ambient temperatures in nature, which has prompted researchers to look for similar sustainable solutions for industrial-scale NH3 synthesis. Photo catalytic ammonia synthesis utilizing sunlight and photo catalysts has received a lot of attention in recent years because it allows for the reducing of N2 to NH3 under very moderate reaction conditions. While photo catalytic ammonia synthesis rates are still far from practical requirements, interesting photo catalysts have already been found, encouraging more study in this field.
Although there are more method to ammonia production, the Haber-Bosch process is the most widely used ammonia production technology, although it has the disadvantages such as high Green House Gases emissions and high energy consumption, owing to its high working pressure and temperature.





Your storytelling abilities are remarkable. You had me captivated from the very first sentence.
This web site is my inhalation, very good style and design and perfect subject material.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.